31. The critical pressure ratio (p2/p1) is given by
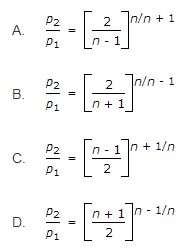
32. In reaction turbines, the axial thrust is due to
A. pressure drop across the rotor
B. change in axial velocity
C. both (a) and (b)
D. none of these
33. Which of the following statement is correct?
A. The expansion of steam in a nozzle follows Rankine cycle.
B. The friction in the nozzle increases the dryness fraction of steam.
C. The pressure of steam at throat is called critical pressure.
D. all of the above
34. A binary vapor plant consists of
A. steam turbine
B. steam condenser
C. mercury boiler
D. all of these
35. In a De-Laval nozzle expanding superheated steam from 10 bar to 0.1 bar, the pressure at the minimum cross-section (i. e. pressure at throat, p2) will be
A.3.3 bar
B.5.46 bar
C.8.2 bar
D.9.9 bar
36. The stage efficiency (ηS) is given by (where ηB = Blading efficiency and ηN = Nozzle efficiency)
A. ηS = ηB x ηN
B. ηS = ηB/ηN
C. ηS = ηN/ηB
D. none of these
37. The critical pressure ratio for initially dry saturated steam is more as compared to initially wet steam.
A. Yes
B. No
38. In a convergent-divergent nozzle, the discharge depends upon the initial conditions of steam and the area of nozzle at throat.
A. Correct
B. Incorrect
39. Parson’s reaction turbine is a __________ reaction turbine.
A. 40 percent
B. 50 percent
C. 60 percent
D. 70 percent
40. The turbine blades are
A. straight
B. circular
C. curved
Warning: Undefined variable $in_same_cat in /www/wwwroot/mtexam.com/public_html/wp-content/plugins/EXP.GKFEED.COM/function.php on line 27
Warning: Undefined variable $excluded_categories in /www/wwwroot/mtexam.com/public_html/wp-content/plugins/EXP.GKFEED.COM/function.php on line 27
